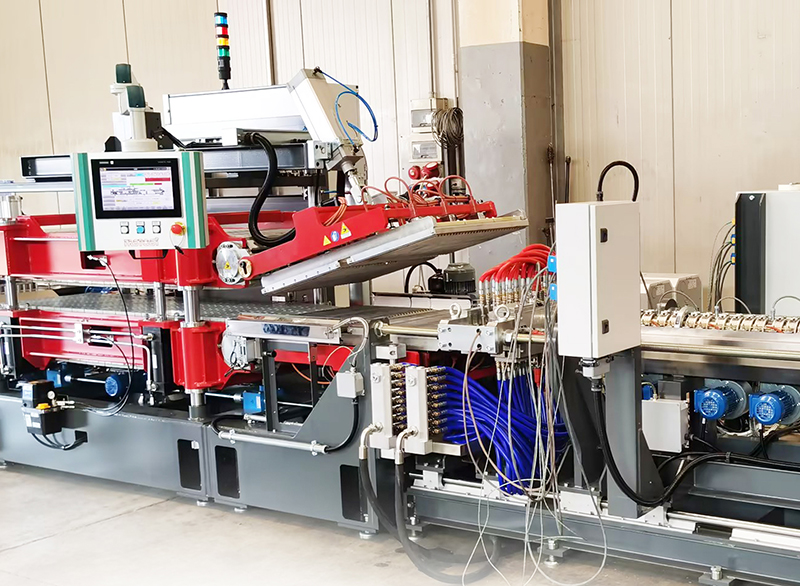
Junhua European imported PEEK rod continuous extrusion production line enters the factory
After a long wait of twelve months, on December 6, Junhua’s second European imported PEEK rod continuous extrusion production line entered the factory. The extrusion is more stable and the production efficiency is higher. It is suitable for large-sca...